金币
UID66198
帖子
主题
积分692
注册时间2012-12-18
最后登录1970-1-1
听众
性别保密
|
欢迎您注册蒲公英
您需要 登录 才可以下载或查看,没有帐号?立即注册
x
FAT & SAT not frequently used as Part of Qualification - ECA Modern Qualification Survey ResultsQualification and Validation regulations have changed in both Europe and the USA in recent years. However, many pharmaceutical companies and suppliers are still using previously practiced methods and documentation - although a risk-based approach has become a regulatory expectation. Also, many companies' activities are not integrated as actually made possible by the EU Annex 15 and the FDA Process Validation guide. So the overall qualification and validation effort is complicated, expensive and time consuming.
“Modern Qualification” is about qualification and validation activities using an integrated approach in close cooperation with suppliers. Yet, frequently suppliers and their documentation are not involved to the extent possible.
Thus, the ECA set up a survey to find out to what extent qualification and validation programmes are integrated as actually made possible by the EU Annex 15 and the FDA Process Validation guide – and to what extent suppliers are involved. Altogether 78 persons provided their feedback. In the following you will find an excerpt of questions and answers provided.
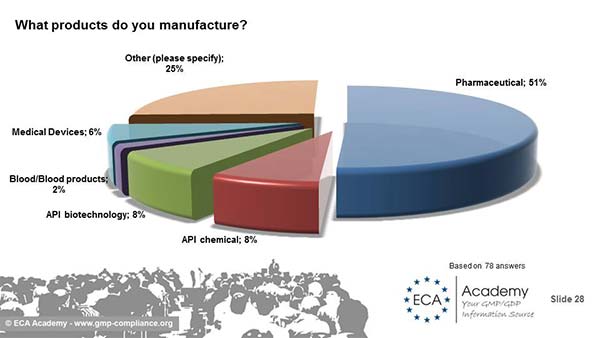
Nearly 75% of the respondents are working for companies with more than 200 employees. Another 15% are with companies with 100-200 employees. 50% of the answers came from colleagues in companies manufacturing pharmaceuticals - and 8% each from API manufacturers in biotechnology and chemical. But there were also answers from Medical Devices manufacturers, equipment suppliers and manufacturers producing a great variety of products (Combination Products, drugs and APIs and Medical Devices).
Most of those responding are involved in validation activities and/or QA (32 participants). Three particpants are from qualification and from project management (each). Two consultants also participated in the survey.
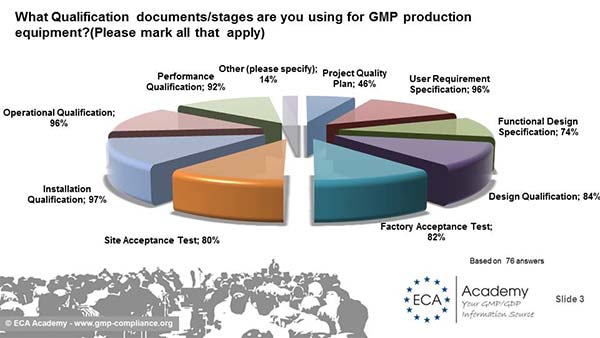
The first question was: What Qualification documents/stages are you using for GMP production equipment?(Please mark all that apply)? Not really surprising: More than 90% are performing IQ/OQ/PQ. Also, User Requirement Specifications are done by more than 96%, Design Qualifcation by 84% and Factory Acceptance Test and Site Acceptance Tests by 82% and 80% resp. 74% do a Functional Design Specification, but only 46% use a Project Plan.
As stated by 82%, their companies have a supplier pre-meeting before they start a qualification project.
Two questions focused on Factory and Site Acceptance Tests: "Are you using FAT/SAT as part of the equipment qualification?" FAT is used by 37% of the companies, 55% stated "it depends on the complecity of the project", and 8% answered with No. SAT is used by 49% and comparable to FAT, 41% answered "it depends on the complecity of the project" and 9% answered with No.
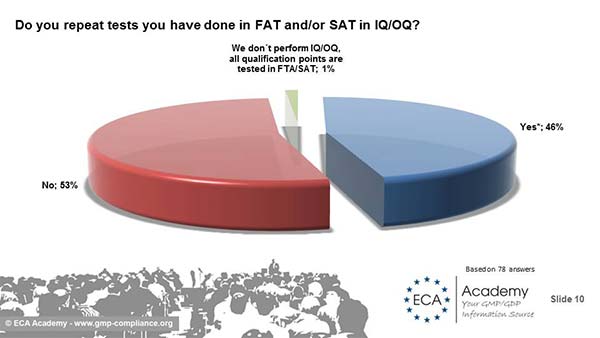
Very interesting are answers to the question: Do you repeat tests you have done in FAT and/or SAT in IQ/OQ? 47% answered with Yes. Why is this so? The main reason is that they do so if transport or equipment installation could have an influence. But there were also answers like "QA department do not allow us to leverage the results", "Because the quality unit is not involved at FAT/SAT..." "it is a purchase process", "We perform specific tests for IQ and OQ"
92% of the participants who answered use supplier documentation in their equipment qualifications.
|
|
 |手机版|蒲公英|ouryao|蒲公英
( 京ICP备14042168号-1 ) 增值电信业务经营许可证编号:京B2-20243455 互联网药品信息服务资格证书编号:(京)-非经营性-2024-0033
|手机版|蒲公英|ouryao|蒲公英
( 京ICP备14042168号-1 ) 增值电信业务经营许可证编号:京B2-20243455 互联网药品信息服务资格证书编号:(京)-非经营性-2024-0033